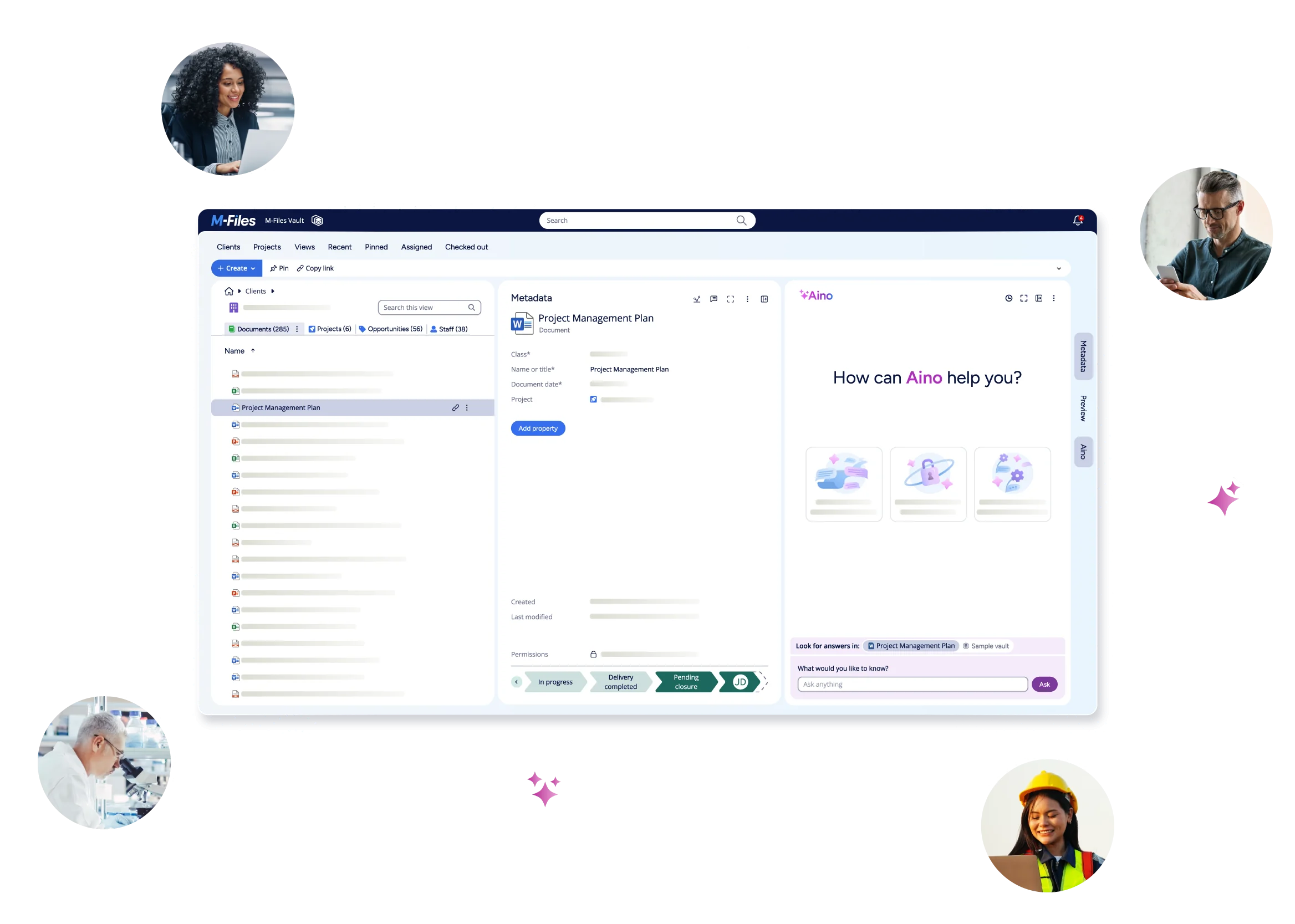
Maximize Research with DMS & Workflow Automation
Revolutionize information management with the leading Knowledge Work Automation Platform. Automate workflows, instantly find and organize client files, strengthen security and compliance, and leverage M-Files GenAI—all in a scalable solution.

Unlock Profit and Growth while Reducing Risk
Improve productivity, quality and profitability of research with better document management.

Automate Documentation of SOPs and CAPAs
- Automate SOPs and resolve CAPAs efficiently through programmed workflows and alerts that provide visibility to deviations from standards
- Meet schedule milestones in conformance with SOPs through review workflows, timelines, reminders, and notifications of delays
- Control quality with workflows, security controls, records management, and approvals automatically implementing your policies
- Increase growth capacity by automating routine tasks so your firm can handle more studies without staff additions
- Improve research associate recruitment and retention by equipping them with tools that reduce their administrative tasks and create more time for science
- Accelerate staff onboarding through templates and workflows that enable them to quickly execute SOPs and handle CAPAs

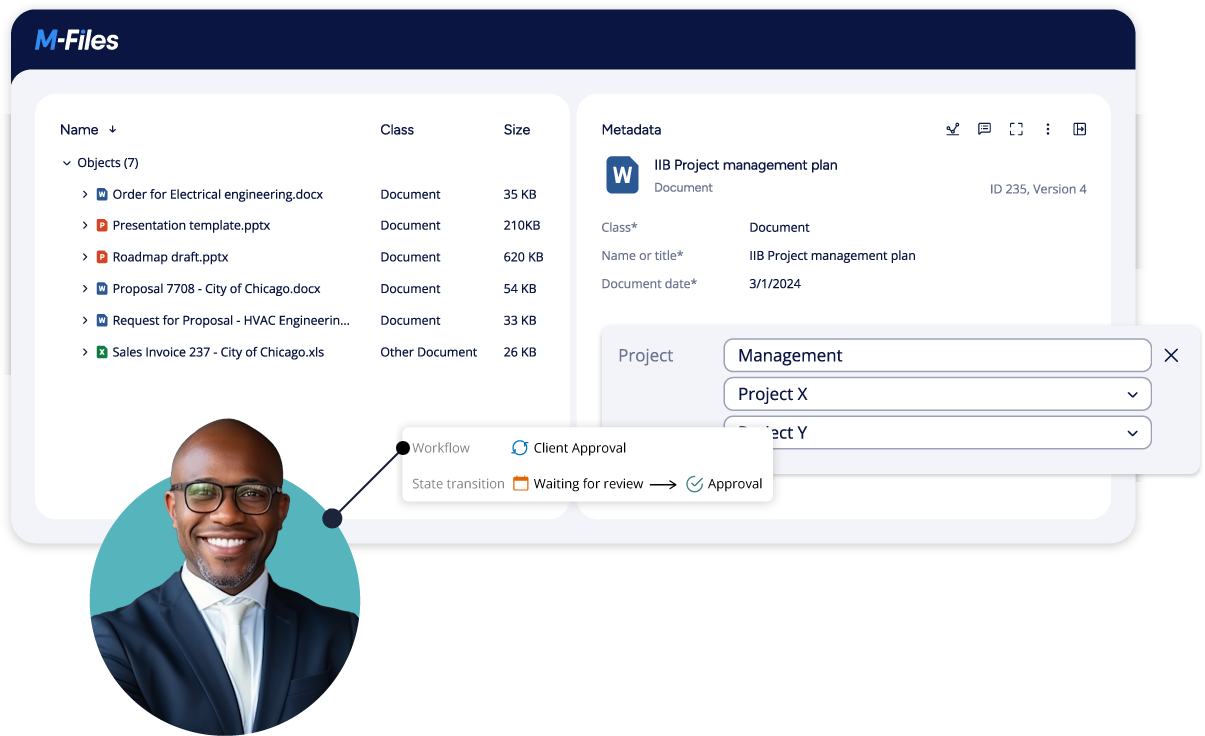
Reduce Business Risk by Ensuring Compliance
- Ensure content security by making documents and data sets available to the right people no matter where or by whom they are stored
- Improve blinding by ensuring that users have zero data visibility unless and until they are identified as approved for access
- Provide evidence of operational oversight that inspires confidence in sponsors and meets regulatory requirements
- Maintain conformance with regulatory requirements through automated SOP execution
- Streamline audits by showing SOP and CAPA controls and record-level tracking of how and when data was generated, approved, and accessed

Streamline Information Management in Scientific Research
- Enhance researcher productivity by streamlining complex documentation tasks, allowing more focus on actual research.
- Ensure document accuracy through consistent application of master data and alerts that flag missing or outdated information.
- Maximize CRM value by making critical research documents easily accessible through primary operational interfaces.
- Improve client acquisition by leveraging past research insights to strengthen proposals and enhance future collaborations.

Strengthen Sponsor Relationships Through Collaborative Workspaces
- Centralize sponsor communications, including inputs, outputs, reviews, and collaborations in a single, secure workspace per project
- Ensure regulatory conformance by tracking when physicians at investigative sites receive, open, and act on critical communications
- Avoid project delays through sponsor workflows, alerts on open tasks, and the ability to collaborate on information in real time
- Avoid losing clinical data that might be overlooked by ensuring version status is not incorrectly coded or miscommunicated
- Track information exchange between investigative sites and sponsors, including acknowledgement of receipt
Work Smarter with M-Files
The M-Files Impact
Up to 294% return on investment with low cost of initial entry and fast time to value.
See M-Files in Action Now
Watch our interactive product tour. No wait. No scheduling.
Start exploring our AI-powered document management system with a demo tailored to your industry.
Already Using Microsoft 365? Let's Make It Work Harder for You.
M-Files delivers pre-built solutions for industries where efficient processes are critical and compliance isn't optional. See how M-Files helps securely organize content, automate workflows, and unlock greater AI value from Microsoft 365.
Learn More About M-Files & Microsoft 365See How The Most Effective Organizations Leverage M-Files
See Context-First Document Management in Action

Industry Use Cases
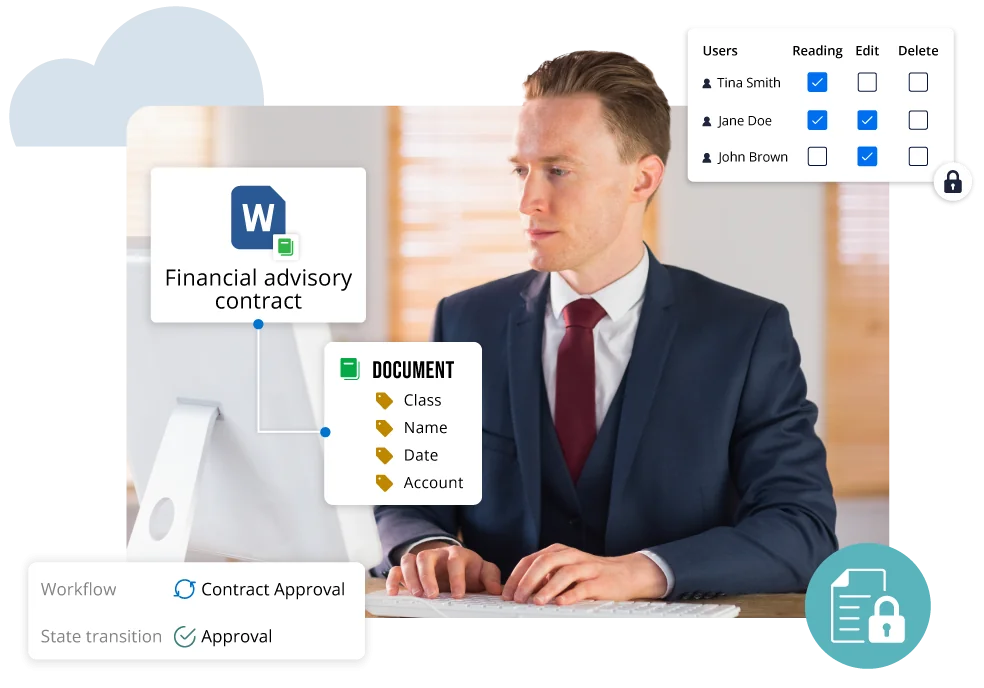
Risk & Compliance Management
- Ensure research data confidentiality and eliminate conflict of interest.
- Manage risk and enforce regulatory compliance.

Quality Management
- Improve quality control and minimize rework.
- Streamline audits with automated evidence from version control and audit trails.
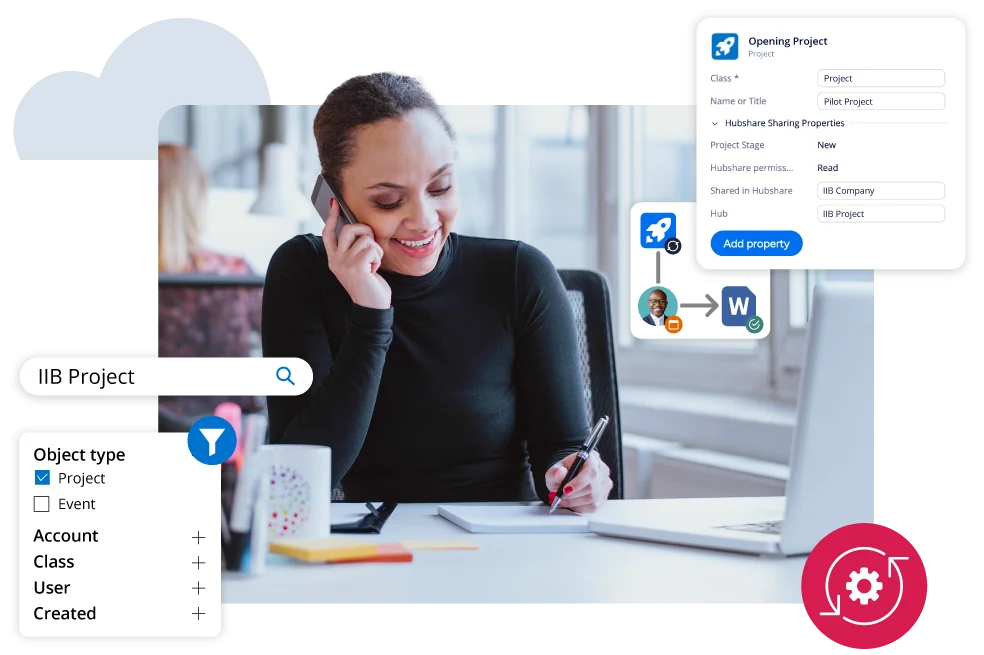
Project Information Management
- Organize information and improve document management.
- Guide processes based on research and project needs.